From Oncology to Immunology to Rare Disease and Beyond
From Oncology to Immunology to Rare Disease and Beyond
Klick’s Team of 185 PhDs, MDs, and PharmDs Drive Success from Molecule to Market.
Klick’s Team of 185 PhDs, MDs, and PharmDs Drive Success from Molecule to Market.
Meet the Mediverse
Meet the Mediverse
![]()
The Klick Mediverse is the world’s largest integrated medical team in the industry. With over 185 experts holding PhDs, MDs, PharmDs, and other advanced degree holders, our team includes writers, strategists, directors, and editors who serve as ambassadors of your science. Together, they form a global center of excellence, delivering comprehensive medical solutions for our clients.
The Klick Mediverse is the world’s largest integrated medical team in the industry. With over 185 experts holding PhDs, MDs, PharmDs, and other advanced degree holders, our team includes writers, strategists, directors, and editors who serve as ambassadors of your science. Together, they form a global center of excellence, delivering comprehensive medical solutions for our clients.
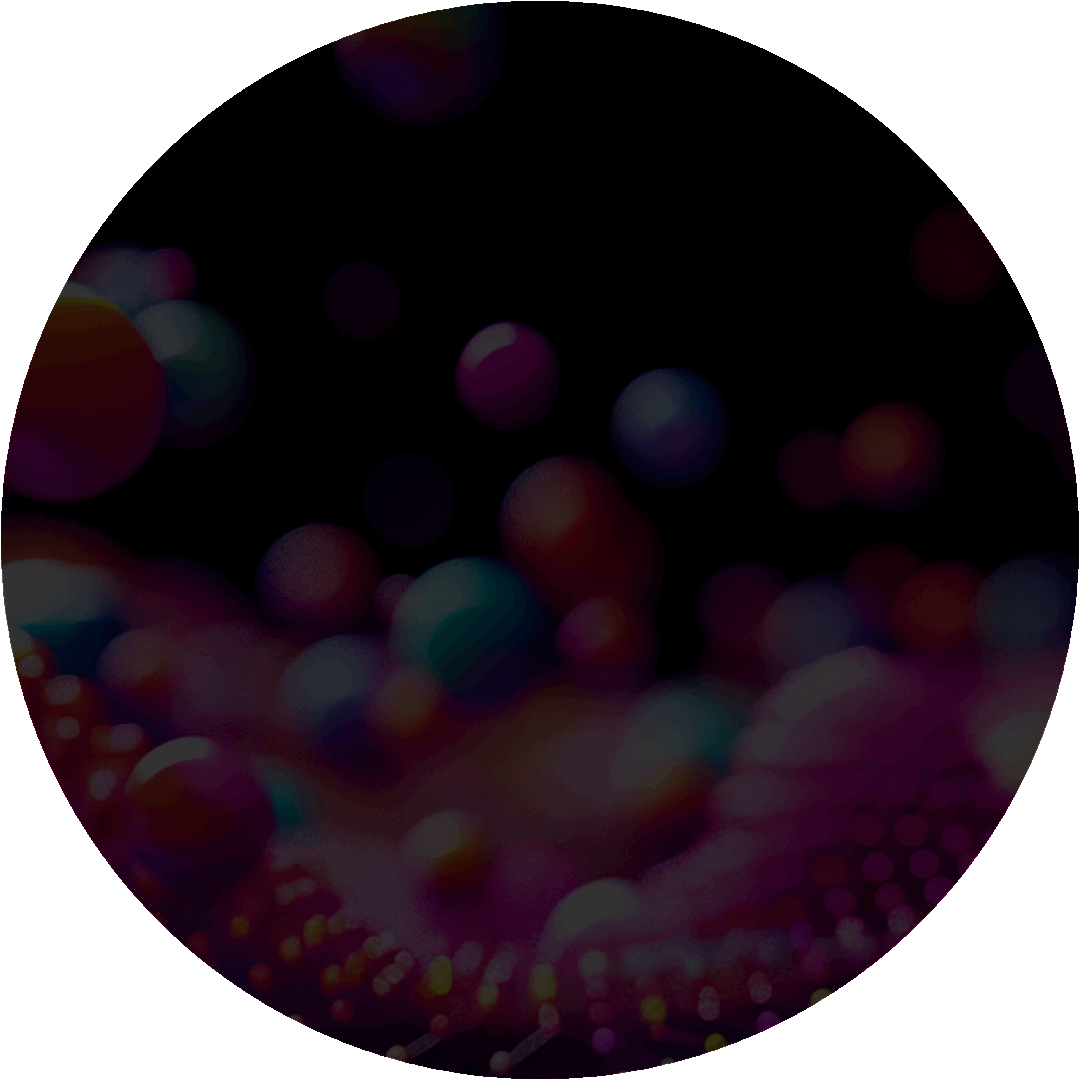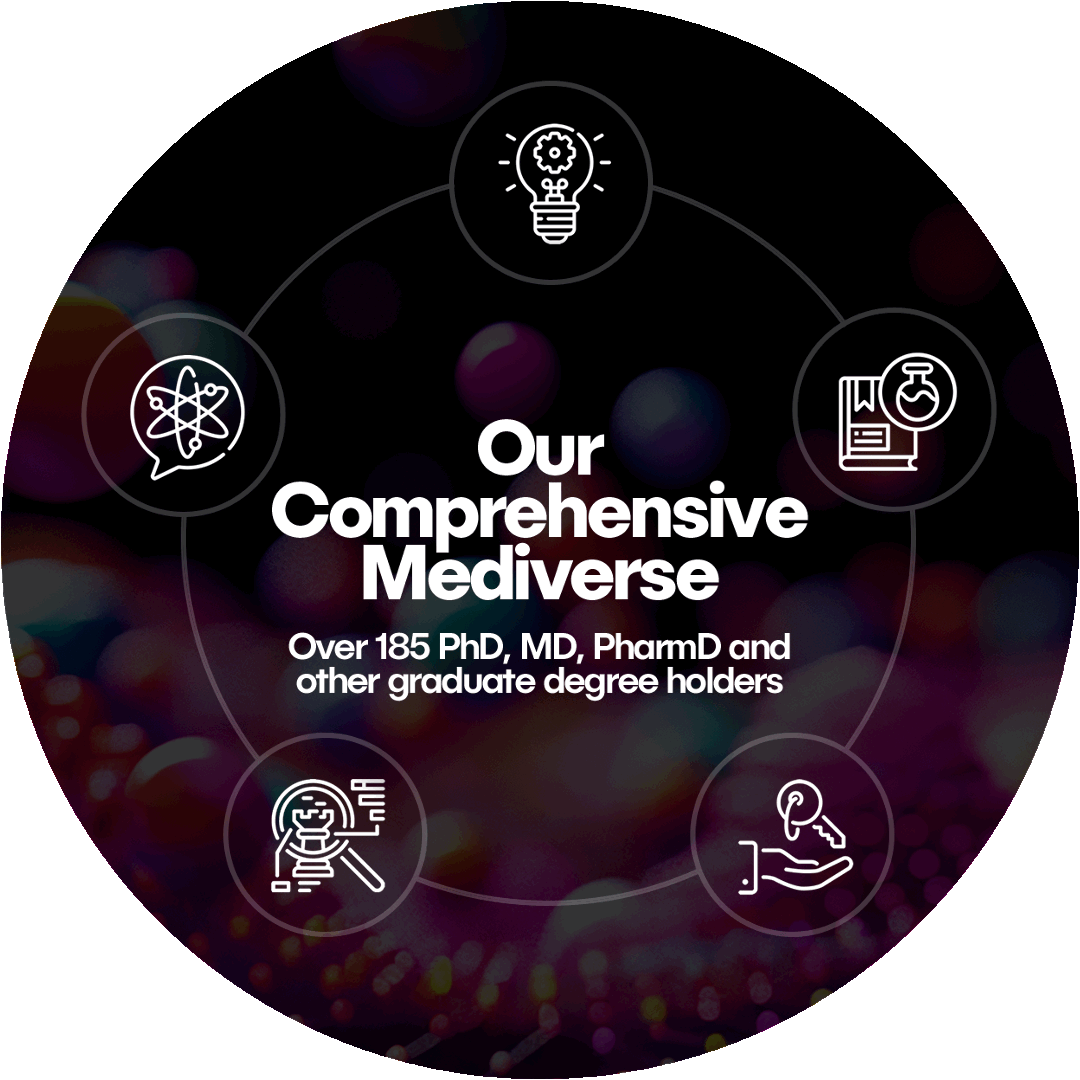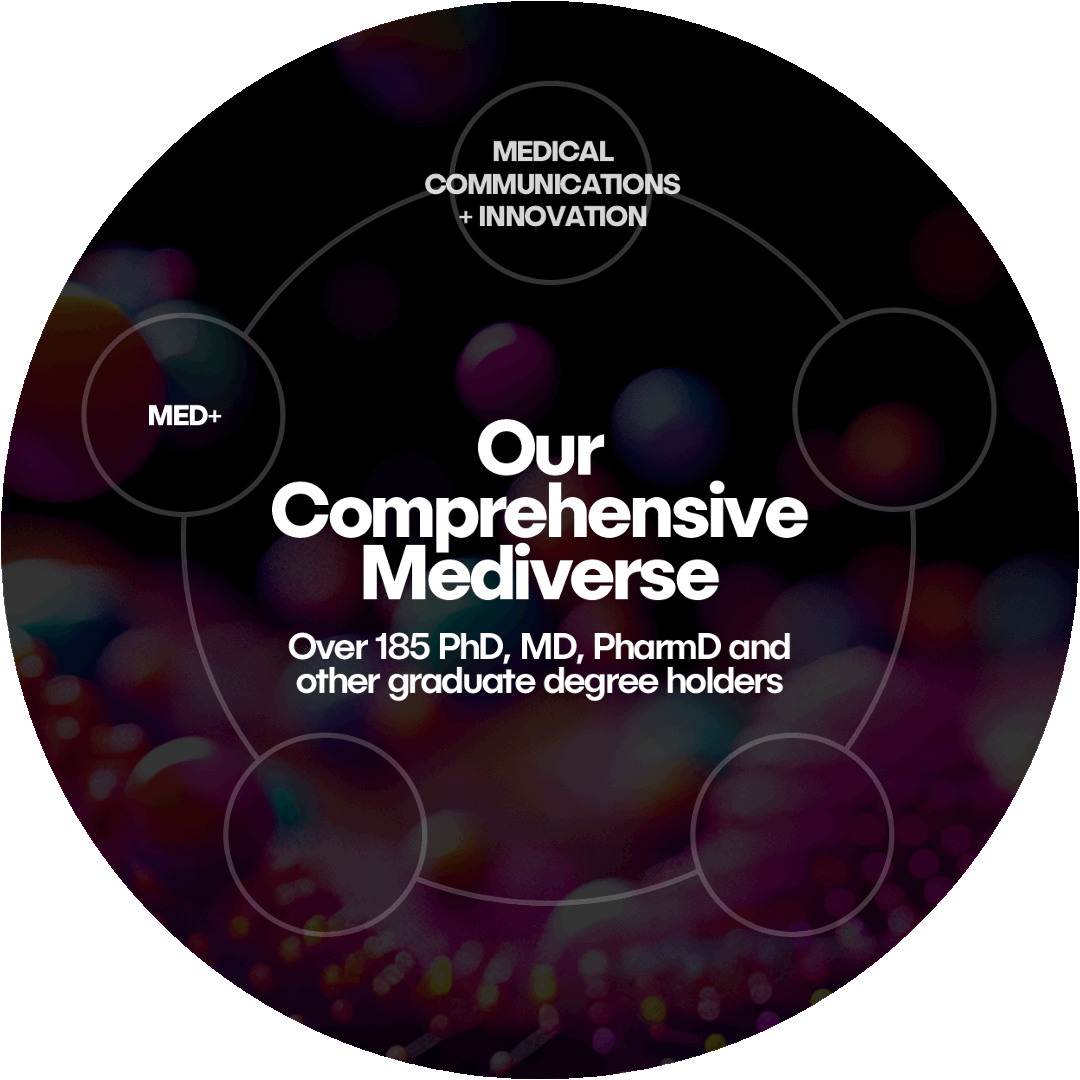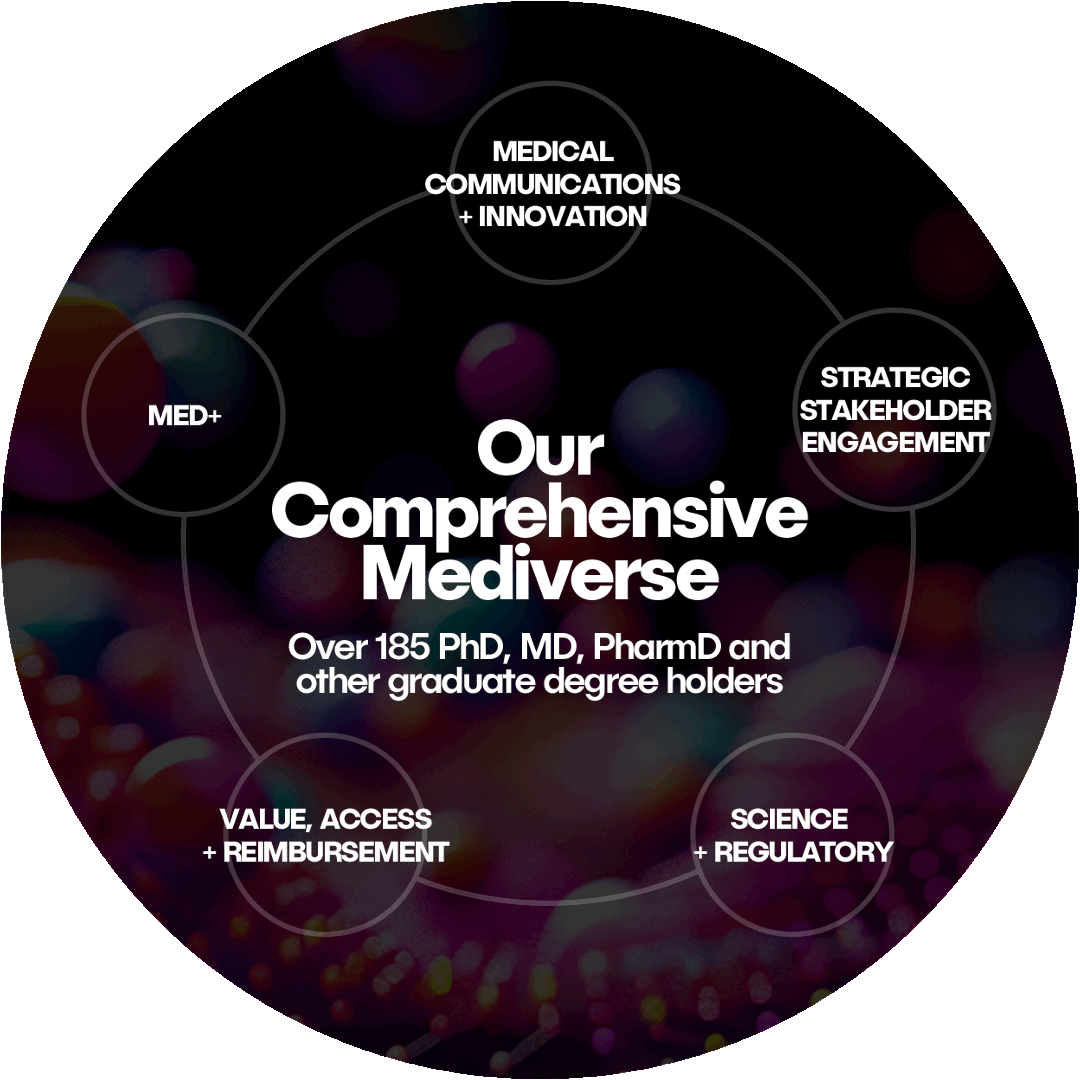
The Mediverse
at a Glance
The Mediverse at a Glance
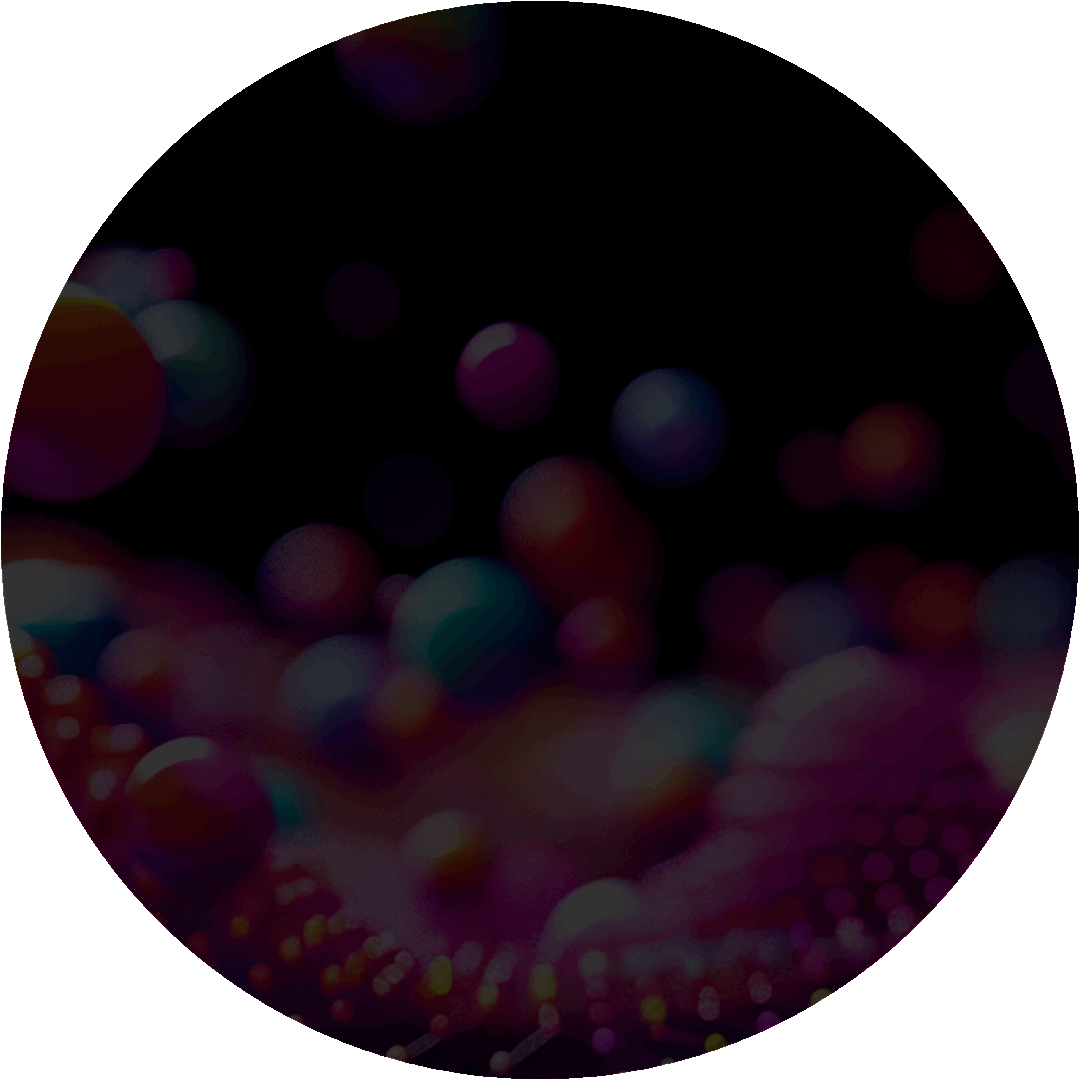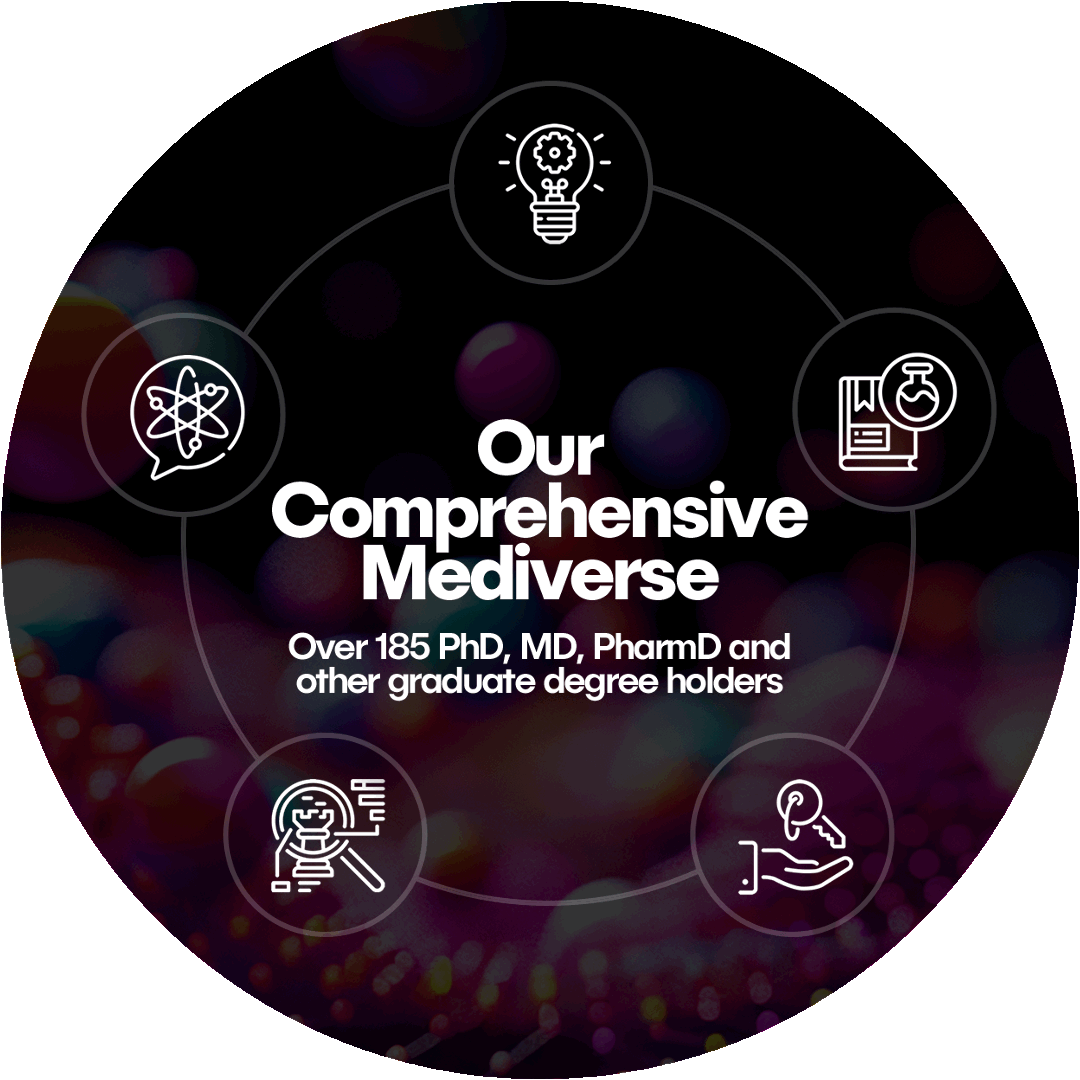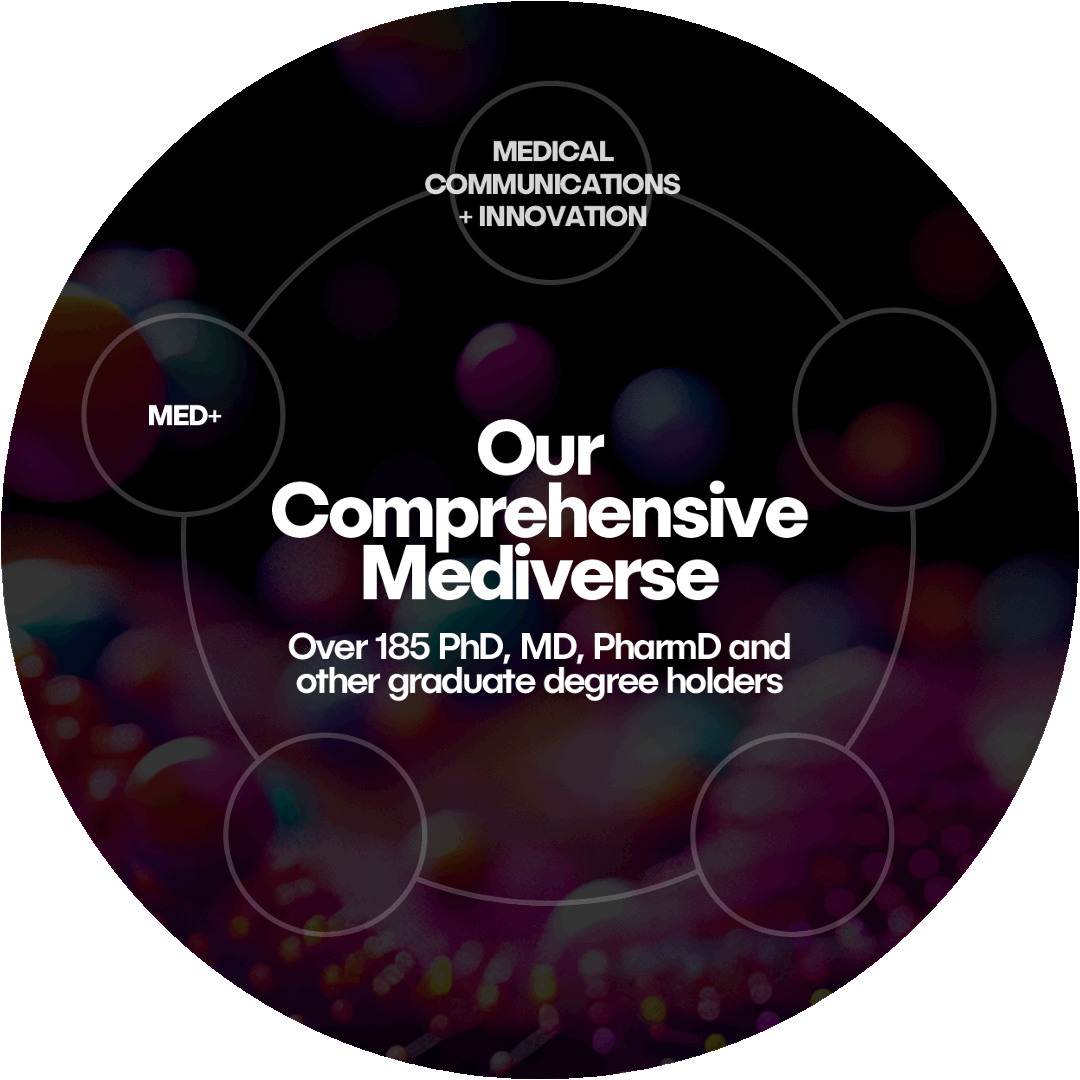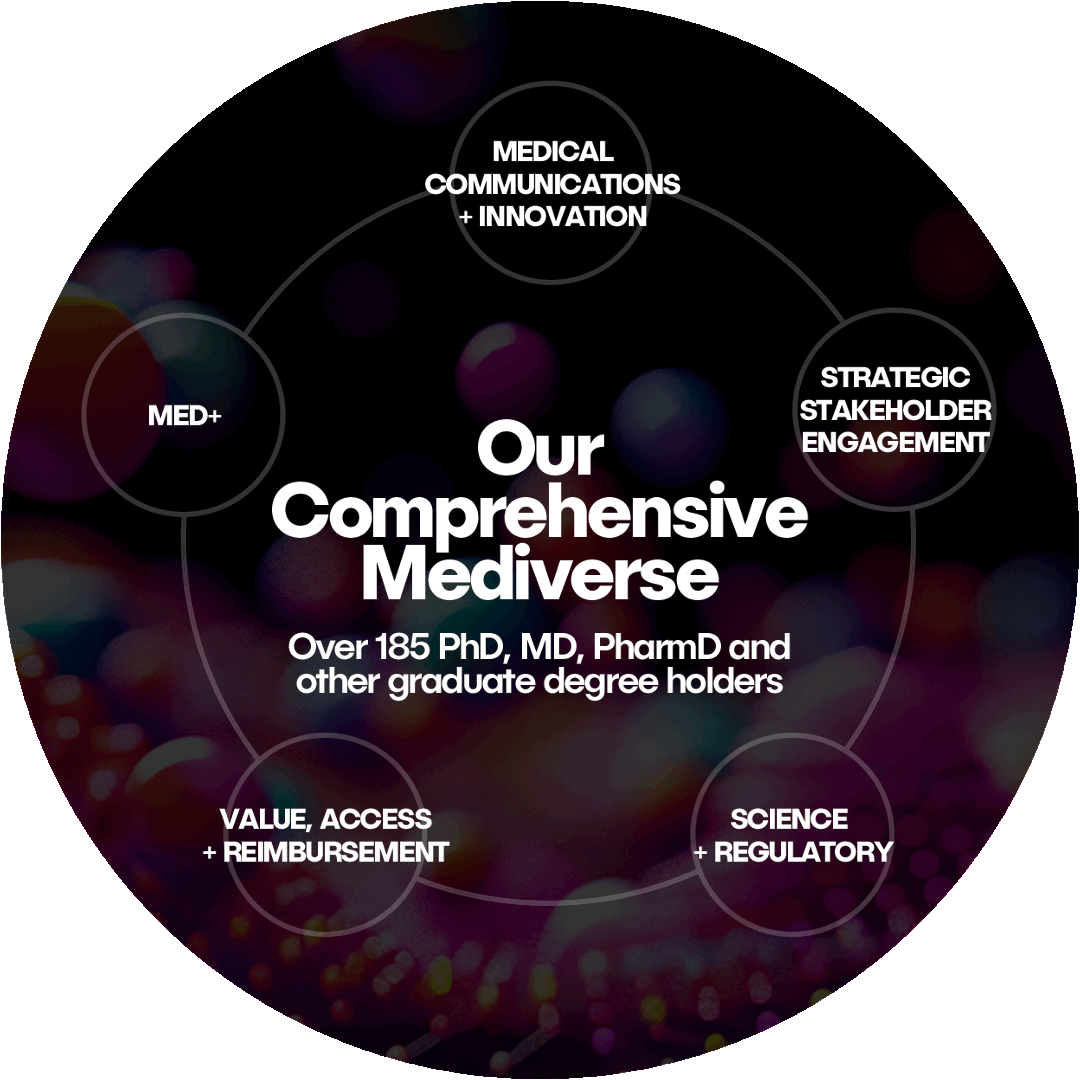
Klick’s integrated approach connects every step of the journey, from early strategy to post-launch support. Each of our five specialized teams seamlessly work together to deliver unparalleled support and innovation for your brand.
The Mediverse in Focus
The Mediverse in Focus
MED+
-
Foundational Medical Support
-
Medical Competitive Assessment
-
Clinical Communication
-
Clinical Trial and Label Optimization
-
Gateway to Mediverse Offerings
Medical Communications + Innovation
-
Medical & Scientific Communications Strategy
-
Omnichannel Strategy
-
Snackable Medical Content
-
DOL/KOL Engagement
-
P2P Innovation
Strategic Stakeholder Engagement
-
Stakeholder Identification and Mapping
-
Stakeholder Engagement and Planning
-
Innovative Stakeholder Solutions
-
Stakeholder Engagement Consultation and Thought Leadership
-
Outcome-Centric Approach
Science + Regulatory
-
Quality + Efficiency
-
MedSci Research + Compliance
-
Regulatory Affairs
-
MLR Relations
-
MLR Ecosystem Management
Value, Access + Reimbursement
-
Insights Driven Access Strategy
-
Value & Evidence-Based Solutions
-
Communication Platform & Deliverables
-
Pull-Through Platforms & Resources
-
Technology-Enabled Solutions
Resources Worth Sharing
Resources Worth Sharing






Breaking Free from “In the Label” Marketing
Navigating FDA regulations in pharmaceutical marketing demands a precise balance between impactful promotion and strict compliance. “Breaking Free from ‘In the Label’ Marketing” offers a detailed guide for aligning marketing strategies with FDA guidance on CFL data. Leveraging Klick’s unmatched expertise in regulatory science, this article provides actionable insights on using real-world data effectively, ensuring that promotional communications remain both compliant and compelling to drive informed decision-making among HCPs and patients. Read more

Navigating the Future of Oncology
The oncology landscape is evolving rapidly, driven by breakthroughs in personalized medicine, AI-powered decision-making, and earlier cancer detection methods. With over 2,300 clinical trials and 700 drugs in late-stage development, oncologists are navigating a wealth of new treatment options alongside emerging challenges. This article explores key trends reshaping cancer care and highlights how Klick’s expertise in applying AI to treatment sequencing, biomarker testing, and personalized therapeutic strategies helps oncologists make more informed, data-driven decisions to improve patient outcomes. Read more

From Molecule to Market:
Take a closer look at Klick Mediverse, where medical, scientific, and regulatory expertise converge to transform healthcare commercialization. Every interaction, from initial drug development to the final market launch, is meticulously crafted to ensure compliance, innovation, and patient care. Discover how Klick's unique approach is converting healthcare challenges into milestones of success and shaping the future of patient care. Read more
Leading Expert Perspectives
Leading Expert Perspectives
Watch in-depth conversations with industry leaders as the Klick Mediverse team explores the latest advancements and insights shaping the future of healthcare.
Mediverse Team Highlights
Mediverse Team Highlights

Holly Henry, PhD
Chief Medical Officer

Joseph Barbagallo, MD
EVP, Head of Medical Strategy

Victoria Sherriff-Scott
EVP, Head of Science + Regulatory

Deirdre McGarrigle, PhD
Executive Director, Medical Strategy

Jasmine Singh, PharmD, MBA
Executive Director, Medical Strategy

Jorge Durand, PhD, MEng
Executive Director, Medical Strategy

Dylan Trent
VP, Science + Regulatory

Julie Turnbull, PhD
VP, Science + Regulatory

Rachael Harrison, PhD
VP, Science + Regulatory

Juhi Chandhok, PharmD
Medical Strategist

Holly Henry, PhD
Chief Medical Officer
As Chief Medical Officer at Klick Health, Holly oversees the world’s largest, fully integrated medical team, ensuring strategic and scientific excellence in communications that support stakeholder education, engagement and ultimately impact patient outcomes. With a background in pharmaceutical and life science companies, Holly has worked across numerous therapeutic areas, focusing on both early clinical development and mature products. Her professional journey began as a Howard Hughes Medical Institute Research Associate, which led to a PhD in cellular and molecular biology from the University of Pennsylvania. Holly’s accolades include a PM360 ELITE Strategist in 2018 and the 2023 MM&M Pinnacle Award.

Joseph Barbagallo, MD
EVP, Head of Medical Strategy
Joseph Barbagallo, Executive Vice President, Head of Medical Strategy at Klick, brings over 20 years of experience in pharmaceutical advertising, along with real-world clinical insights as a board-certified internist. He specializes in aligning strategic and scientific communications, playing a pivotal role in leading US and global brand initiatives across a wide range of therapeutic areas such as rheumatology, dermatology, neurology, psychiatry, oncology, nephrology, CV and GI diseases. His expertise covers the entire product life cycle, from pre-launch/commercialization strategy to post-market success, ensuring campaigns are grounded in clinical insights and meet regulatory and commercial objectives.
Before joining Klick, Joe held senior medical roles at FCB Health and CDM Group in New York City, where he became known for combining clinical expertise with strategic marketing. Joe earned a BS from Cornell University, an MD from SUNY Health Science Center at Syracuse, and completed his residency in internal medicine at Columbia University. He has also authored several publications in peer-reviewed journals.

Victoria Sherriff-Scott
EVP, Head of Science + Regulatory
Victoria has been instrumental in growing the capabilities of a diverse, multi-service team, effectively establishing a new offering in the ad promo industry with a unique blend of efficiency, expertise, and value: Science + Regulatory. By introducing new skill sets, tools, and processes in medical editing, regulatory compliance, medsci research, MLR submission management, and risk mitigation, Victoria rapidly expanded the team’s focus, capabilities, and impact. The team leverages their scientific and regulatory expertise to partner with clients throughout commercialization, guiding brands from ideation and planning to execution, MLR approval, and launch.
Victoria brings the same drive for differentiation and growth as a key leader of Klick’s Medical Group, the largest integrated medical team within a full-service agency worldwide. She is uniquely positioned to consult on quality, accuracy, and compliance in the medical and regulatory space for US, Canadian, and global clients.

Deirdre McGarrigle, PhD
Executive Director, Medical Strategy
Deirdre brings over 15 years of experience in medical strategy to her role at Klick. She has worked across multiple therapeutic categories and product lifecycle stages including navigating the complexities of rare diseases such as pediatric neuroblastoma, cystic fibrosis, growth hormone deficiency, lysosomal storage disorders and lupus nephritis. She is dedicated to improving patient outcomes by developing strategies that drive awareness, education, and innovative solutions to meet the unique challenges of underserved conditions. Deirdre holds a first-class honours degree in Physiology from University College Dublin, Ireland, and a PhD in Physiology, Biophysics, and Molecular Medicine from Cornell University’s Weill Graduate School of Medical Sciences.

Jasmine Singh, PharmD, MBA
Executive Director, Medical Strategy
As a clinical pharmacist, Jasmine brings real-world insights across a range of disease states and patient populations, ensuring relevance and credibility in disseminating data. She has guided high-profile therapies through all phases of drug development and life cycle management, including launching new products, supporting new indications, and repositioning mature brands.
Jasmine’s oncology experience spans supportive care treatments like PROCRIT for anemia associated with various tumor types to targeted therapies like AVASTIN, where she served as the medical lead for its launch in glioblastoma. Most recently, Jasmine led the launch of QINLOCK for GIST, overseeing disease state awareness, patient/treatment journey mapping, and KOL engagement. Jasmine has also supported brands in both oncology and hematology, including DOXIL, VELCADE, FARYDAK, FEMARA, XELODA, REVLIMID, and IBRANCE. With additional experience in rare tumor types, Jasmine is recognized as an oncology subject matter expert and a valuable member of Klick’s medical strategy team.

Jorge Durand, PhD, MEng
Executive Director, Medical Strategy
With over 15 years working as a medical strategist, Jorge has helped build healthcare brands in the US and globally, including oncology, immunology, and rare disease, among others. Jorge has led marketing and medical affairs efforts, from the development of branded and unbranded campaigns to optimizing UX and omnichannel strategies. He has also fostered collaboration with thought leaders and patient advocate organizations, especially in brands that have a significant impact on patients’ lives. Since joining Klick in 2017, Jorge has helped expand our client roster as well as our medical strategy team from 3 to currently almost 30.
Jorge graduated with a BS and an MS in nuclear engineering from Balseiro Institute (Argentina). He completed his PhD/MS in biomedical sciences at Albert Einstein College of Medicine, where he also did postdoctoral research.

Dylan Trent
VP, Science + Regulatory
Dylan is a pharmaceutical marketing veteran with almost 15 years of experience in the creation, review, and submission of highly-regulated advertising and promotional materials for dozens of top pharmaceutical companies. At Klick, Dylan leads a team of medical and regulatory experts who are adept at delivering quality, innovative content for groundbreaking launches and award-winning campaigns that push the boundaries for clients worldwide.
Before joining Klick in 2019, Dylan founded and grew a regulatory team that spanned a network of agencies serving global clients. In 2022, Dylan was honored with MM&M’s 40 Under 40 award for his contributions and progressive approach to advancing the life sciences industry.

Julie Turnbull, PhD
VP, Science + Regulatory
As Vice President of Science + Regulatory, Julie Turnbull expertly leads the team in ensuring medical quality and regulatory excellence. With over 20 years of healthcare experience, including 10 years specializing in rare disorders, Julie has led research teams working with rare disease patients, witnessing firsthand the transformative impact of new treatments on this community. Julie has authored over 35 peer-reviewed publications in neurology and rare diseases and has presented as an expert to both HCP and patient audiences. Over the past 10 years at Klick Health, she has directly impacted numerous rare disease campaigns and launches for HCPs and patients alike, and is passionate about making a difference in this community.

Rachael Harrison, PhD
VP, Science + Regulatory
As a Vice President of Science + Regulatory at Klick, Rachael manages many departments of Medical and Regulatory Editors and several client portfolios. She has a PhD in Physiology, and her postdoctoral work focused specifically on glucose and fat metabolism (in first Oncology, and later Trauma patients). Since transitioning from translational research into healthcare marketing and regulatory consulting over a decade ago, Rachael has continued to expand her experience into multiple disease states/indications, including Metabolism/Obesity, Vaccines, Transplant, Renal disorders, Infectious Diseases/Antibiotics, Autoimmune Disorders, Men’s Health, Diabetes, and Neurology.

Juhi Chandhok, PharmD
Medical Strategist
Juhi brings over 5 years of expertise in promotional advertising, with a strong focus on the Obesity space, where she led the launch of ZEPBOUND/MOUNJARO. For four years, she managed all US, global, and DTC/HCP accounts, guiding the brands' medical strategy from prelaunch to a successful launch.

Holly Henry, PhD
Chief Medical Officer

Joseph Barbagallo, MD
EVP, Head of Medical Strategy

Victoria Sherriff-Scott
EVP, Head of Science + Regulatory

Deirdre McGarrigle, PhD
Executive Director, Medical Strategy

Jasmine Singh, PharmD, MBA
Executive Director, Medical Strategy

Jorge Durand, PhD, MEng
Executive Director, Medical Strategy

Dylan Trent
VP, Science + Regulatory

Julie Turnbull, PhD
VP, Science + Regulatory

Rachael Harrison, PhD
VP, Science + Regulatory

Juhi Chandhok, PharmD
Medical Strategist

Holly Henry, PhD
Chief Medical Officer
As Chief Medical Officer at Klick Health, Holly oversees the world’s largest, fully integrated medical team, ensuring strategic and scientific excellence in communications that support stakeholder education, engagement and ultimately impact patient outcomes. With a background in pharmaceutical and life science companies, Holly has worked across numerous therapeutic areas, focusing on both early clinical development and mature products. Her professional journey began as a Howard Hughes Medical Institute Research Associate, which led to a PhD in cellular and molecular biology from the University of Pennsylvania. Holly’s accolades include a PM360 ELITE Strategist in 2018 and the 2023 MM&M Pinnacle Award.

Joseph Barbagallo, MD
EVP, Head of Medical Strategy
Joseph Barbagallo, Executive Vice President, Head of Medical Strategy at Klick, brings over 20 years of experience in pharmaceutical advertising, along with real-world clinical insights as a board-certified internist. He specializes in aligning strategic and scientific communications, playing a pivotal role in leading US and global brand initiatives across a wide range of therapeutic areas such as rheumatology, dermatology, neurology, psychiatry, oncology, nephrology, CV and GI diseases. His expertise covers the entire product life cycle, from pre-launch/commercialization strategy to post-market success, ensuring campaigns are grounded in clinical insights and meet regulatory and commercial objectives.
Before joining Klick, Joe held senior medical roles at FCB Health and CDM Group in New York City, where he became known for combining clinical expertise with strategic marketing. Joe earned a BS from Cornell University, an MD from SUNY Health Science Center at Syracuse, and completed his residency in internal medicine at Columbia University. He has also authored several publications in peer-reviewed journals.

Victoria Sherriff-Scott
EVP, Head of Science + Regulatory
Victoria has been instrumental in growing the capabilities of a diverse, multi-service team, effectively establishing a new offering in the ad promo industry with a unique blend of efficiency, expertise, and value: Science + Regulatory. By introducing new skill sets, tools, and processes in medical editing, regulatory compliance, medsci research, MLR submission management, and risk mitigation, Victoria rapidly expanded the team’s focus, capabilities, and impact. The team leverages their scientific and regulatory expertise to partner with clients throughout commercialization, guiding brands from ideation and planning to execution, MLR approval, and launch.
Victoria brings the same drive for differentiation and growth as a key leader of Klick’s Medical Group, the largest integrated medical team within a full-service agency worldwide. She is uniquely positioned to consult on quality, accuracy, and compliance in the medical and regulatory space for US, Canadian, and global clients.

Deirdre McGarrigle, PhD
Executive Director, Medical Strategy
Deirdre brings over 15 years of experience in medical strategy to her role at Klick. She has worked across multiple therapeutic categories and product lifecycle stages including navigating the complexities of rare diseases such as pediatric neuroblastoma, cystic fibrosis, growth hormone deficiency, lysosomal storage disorders and lupus nephritis. She is dedicated to improving patient outcomes by developing strategies that drive awareness, education, and innovative solutions to meet the unique challenges of underserved conditions. Deirdre holds a first-class honours degree in Physiology from University College Dublin, Ireland, and a PhD in Physiology, Biophysics, and Molecular Medicine from Cornell University’s Weill Graduate School of Medical Sciences.

Jasmine Singh, PharmD, MBA
Executive Director, Medical Strategy
As a clinical pharmacist, Jasmine brings real-world insights across a range of disease states and patient populations, ensuring relevance and credibility in disseminating data. She has guided high-profile therapies through all phases of drug development and life cycle management, including launching new products, supporting new indications, and repositioning mature brands.
Jasmine’s oncology experience spans supportive care treatments like PROCRIT for anemia associated with various tumor types to targeted therapies like AVASTIN, where she served as the medical lead for its launch in glioblastoma. Most recently, Jasmine led the launch of QINLOCK for GIST, overseeing disease state awareness, patient/treatment journey mapping, and KOL engagement. Jasmine has also supported brands in both oncology and hematology, including DOXIL, VELCADE, FARYDAK, FEMARA, XELODA, REVLIMID, and IBRANCE. With additional experience in rare tumor types, Jasmine is recognized as an oncology subject matter expert and a valuable member of Klick’s medical strategy team.

Jorge Durand, PhD, MEng
Executive Director, Medical Strategy
With over 15 years working as a medical strategist, Jorge has helped build healthcare brands in the US and globally, including oncology, immunology, and rare disease, among others. Jorge has led marketing and medical affairs efforts, from the development of branded and unbranded campaigns to optimizing UX and omnichannel strategies. He has also fostered collaboration with thought leaders and patient advocate organizations, especially in brands that have a significant impact on patients’ lives. Since joining Klick in 2017, Jorge has helped expand our client roster as well as our medical strategy team from 3 to currently almost 30.
Jorge graduated with a BS and an MS in nuclear engineering from Balseiro Institute (Argentina). He completed his PhD/MS in biomedical sciences at Albert Einstein College of Medicine, where he also did postdoctoral research.

Dylan Trent
VP, Science + Regulatory
Dylan is a pharmaceutical marketing veteran with almost 15 years of experience in the creation, review, and submission of highly-regulated advertising and promotional materials for dozens of top pharmaceutical companies. At Klick, Dylan leads a team of medical and regulatory experts who are adept at delivering quality, innovative content for groundbreaking launches and award-winning campaigns that push the boundaries for clients worldwide.
Before joining Klick in 2019, Dylan founded and grew a regulatory team that spanned a network of agencies serving global clients. In 2022, Dylan was honored with MM&M’s 40 Under 40 award for his contributions and progressive approach to advancing the life sciences industry.

Julie Turnbull, PhD
VP, Science + Regulatory
As Vice President of Science + Regulatory, Julie Turnbull expertly leads the team in ensuring medical quality and regulatory excellence. With over 20 years of healthcare experience, including 10 years specializing in rare disorders, Julie has led research teams working with rare disease patients, witnessing firsthand the transformative impact of new treatments on this community. Julie has authored over 35 peer-reviewed publications in neurology and rare diseases and has presented as an expert to both HCP and patient audiences. Over the past 10 years at Klick Health, she has directly impacted numerous rare disease campaigns and launches for HCPs and patients alike, and is passionate about making a difference in this community.

Rachael Harrison, PhD
VP, Science + Regulatory
As a Vice President of Science + Regulatory at Klick, Rachael manages many departments of Medical and Regulatory Editors and several client portfolios. She has a PhD in Physiology, and her postdoctoral work focused specifically on glucose and fat metabolism (in first Oncology, and later Trauma patients). Since transitioning from translational research into healthcare marketing and regulatory consulting over a decade ago, Rachael has continued to expand her experience into multiple disease states/indications, including Metabolism/Obesity, Vaccines, Transplant, Renal disorders, Infectious Diseases/Antibiotics, Autoimmune Disorders, Men’s Health, Diabetes, and Neurology.

Juhi Chandhok, PharmD
Medical Strategist
Juhi brings over 5 years of expertise in promotional advertising, with a strong focus on the Obesity space, where she led the launch of ZEPBOUND/MOUNJARO. For four years, she managed all US, global, and DTC/HCP accounts, guiding the brands' medical strategy from prelaunch to a successful launch.


